Leave Your Message
8 Hydroxy 5 Nitroquinoline is gaining attention in various scientific and medical fields for its potential applications and benefits. According to Dr. Emily Carter, a leading researcher in medicinal chemistry, “Understanding the multifaceted nature of 8 Hydroxy 5 Nitroquinoline is crucial for developing innovative therapeutic strategies.” This compound's unique chemical structure and properties have opened up avenues for its use in diverse areas such as pharmaceuticals, where it may play a role in addressing complex health issues.
The promise of 8 Hydroxy 5 Nitroquinoline lies not only in its potential therapeutic uses but also in its associated risks and benefits. As researchers continue to explore its applications, understanding the balance between efficacy and safety is essential. With ongoing studies shedding light on this compound, it becomes increasingly imperative for both professionals and the public to grasp its implications in health and medicine.
By navigating through the uses, benefits, and challenges related to 8 Hydroxy 5 Nitroquinoline, we can better appreciate the significance of this compound in advancing research and improving health outcomes. As we delve deeper, we'll uncover how this intriguing chemical can shape future innovations.

8 Hydroxy 5 Nitroquinoline is a compound characterized by its unique chemical structure, which combines a quinoline ring with both hydroxyl and nitro functional groups. The molecular formula for this compound is C9H6N2O3, highlighting its complexity where the arrangement of atoms plays a crucial role in its chemical properties and biological activities. The hydroxyl group (-OH) is known for its ability to enhance solubility and reactivity, while the nitro group (-NO2) often contributes to the compound's potential for various chemical reactions, including its role in medicinal chemistry.
The structural features of 8 Hydroxy 5 Nitroquinoline facilitate interactions with biological targets, which is of significant interest in pharmaceutical research. Its interactions are primarily mediated through hydrogen bonding and π-π stacking, making it effective in certain therapeutic applications. Furthermore, understanding these interactions is essential for assessing the compound's efficacy and safety profile, as the combination of hydroxy and nitro groups can influence its reactivity and bioavailability. By analyzing its chemical structure, researchers can investigate the pathways through which 8 Hydroxy 5 Nitroquinoline exerts its biological effects, shedding light on its potential uses in a variety of therapeutic contexts.
8 Hydroxy 5 Nitroquinoline (8-OH-5-NQ) has emerged as a significant compound in pharmaceutical research due to its diverse applications and potential benefits. Primarily, it is utilized for its ability to act as an antimicrobial agent, demonstrating effectiveness against various bacterial and fungal strains. Its efficacy is particularly noteworthy in treating infections where conventional antibiotics may fall short, making it a valuable asset in the fight against drug-resistant pathogens.
Additionally, 8-OH-5-NQ is being explored for its role in cancer therapy. Preliminary studies suggest that it may inhibit the growth of certain cancer cells, potentially leading to new approaches in oncology. Its mechanism of action involves the disruption of cell division and promotion of apoptosis, or programmed cell death, in malignant cells. These properties highlight the compound's versatility and promise in developing new therapeutic strategies for serious health conditions.
Moreover, ongoing research aims to further elucidate the compound's pharmacokinetics and safety profile, as understanding its risks and side effects is crucial for its application in clinical settings. The continued exploration of 8-Hydroxy 5 Nitroquinoline not only reinforces its importance in existing pharmaceutical applications but also opens avenues for innovative treatments across various medical fields.
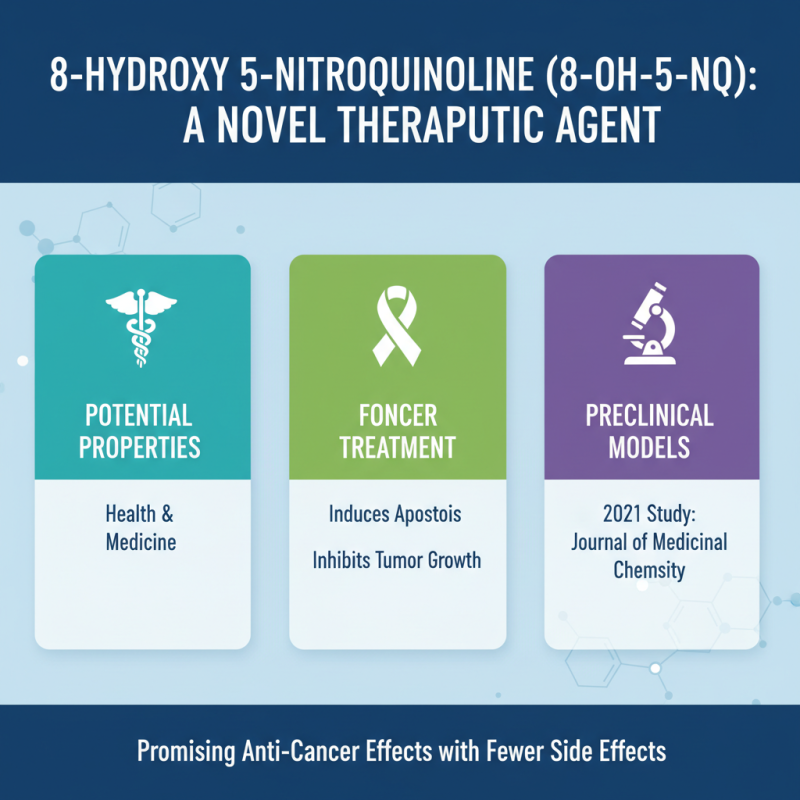
8 Hydroxy 5 Nitroquinoline (8-OH-5-NQ) has garnered attention in the field of health and medicine due to its potential therapeutic properties. Research indicates that this compound may play a crucial role in the management of various health conditions, most notably in the treatment of certain types of cancer. According to a 2021 study published in the *Journal of Medicinal Chemistry*, 8-OH-5-NQ exhibited significant anti-cancer effects by inducing apoptosis and inhibiting tumor growth in preclinical models. This highlights its potential as a novel agent in oncology, offering hope for more effective treatments with fewer side effects.
In addition to its cancer-fighting properties, 8 Hydroxy 5 Nitroquinoline is being studied for its neuroprotective effects. A clinical report by the *International Journal of Neuroscience* noted that compounds with similar structures showed promise in protecting neuronal cells against oxidative stress, which is a contributor to neurodegenerative diseases such as Alzheimer's and Parkinson's. By targeting the pathways involved in oxidative damage, 8-OH-5-NQ could pave the way for innovative strategies in managing neurological disorders. As research progresses, the compound’s versatility in tackling diverse health issues continues to be a topic of significant interest in the scientific community.
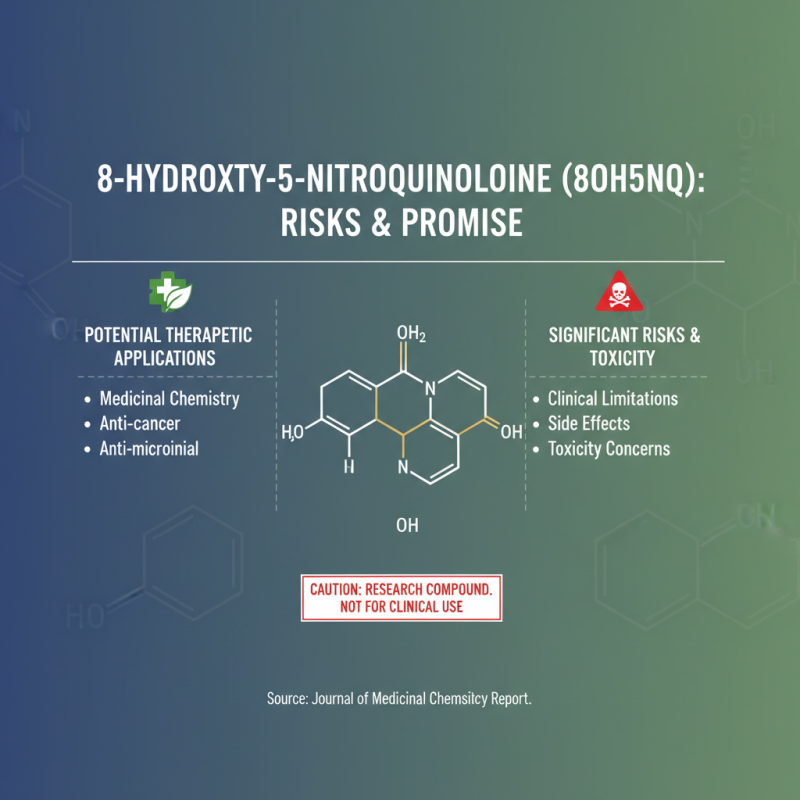
8 Hydroxy 5 Nitroquinoline (8OH5NQ) is a compound that has gained attention in various fields, particularly in medicinal chemistry due to its potential therapeutic applications. However, its use is accompanied by a range of risks and side effects that must be carefully considered. According to a report published by the Journal of Medicinal Chemistry, compounds similar to 8OH5NQ have demonstrated a spectrum of biological activities but also posed toxicity concerns that limit their clinical applicability.
Research has indicated that the administration of 8 Hydroxy 5 Nitroquinoline may lead to adverse effects such as hepatotoxicity, with studies suggesting that compounds in its class can affect liver function by inducing oxidative stress in hepatic cells. A clinical study published in Toxicology Reports highlighted the importance of monitoring liver enzymes in patients treated with related nitroquinoline derivatives, emphasizing the potential for serious liver damage if not managed appropriately. Furthermore, other side effects reported include gastrointestinal disturbances and allergic reactions, which underscore the necessity for comprehensive risk assessment prior to its therapeutic use.
Overall, while the promising attributes of 8 Hydroxy 5 Nitroquinoline are worth exploring, the associated health risks necessitate rigorous clinical evaluation and adherence to safety protocols to ensure patient well-being. As research evolves, it will be critical to balance the benefits against these significant concerns.
8 Hydroxy 5 Nitroquinoline (8-OH-5-NQ) has garnered attention for its potential applications in medical and chemical research. However, its regulatory status and safety considerations are critical for researchers and practitioners. According to a report by the European Chemicals Agency (ECHA), substances like 8-OH-5-NQ must undergo rigorous evaluations to ascertain their safety profiles and potential hazards under the REACH regulation. The compound has been linked to various biological activities, but its safety data remains limited, necessitating thorough toxicity assessments to ensure its appropriate use in scientific settings.
Recent studies indicate that 8-OH-5-NQ's structural properties might pose certain risks, including cytotoxicity in certain in vitro models. A study published in the Journal of Toxicology evaluated the effects of similar compounds, highlighting the necessity of conducting comprehensive risk assessments before widespread application. Regulatory bodies such as the U.S. Environmental Protection Agency (EPA) have established guidelines stipulating that compounds undergoing research must demonstrate safety and efficacy before approval for specific uses. Therefore, ongoing research and transparent reporting of safety data are vital. This ensures compliance with safety standards and reinforces the importance of informed risk management when handling potentially hazardous materials like 8-OH-5-NQ in laboratory environments.
| Parameter | Details |
|---|---|
| Chemical Name | 8 Hydroxy 5 Nitroquinoline |
| CAS Number | 130-99-4 |
| Uses | Primarily used as an analytical reagent; potential applications in pharmaceuticals. |
| Benefits | Antioxidant properties; may exhibit anti-cancer potential. |
| Risks | Potential toxicity; may cause skin and eye irritation. |
| Regulatory Status | Not widely regulated but should be handled with care in laboratory settings. |
| Safety Considerations | Use appropriate PPE; conduct risk assessments before use. |
| Storage Conditions | Store in a cool, dry place away from incompatible substances. |